Notice of the General Administration of Market Supervision on Printing and Distributing the Development Plan for Certification, Accreditation, Inspection and Testing during the 14th Five-Year Plan
General Administration of Market Supervision on IssuingNotice of the Development Plan for Certification, Accreditation, Inspection and Testing in the 14th Five-Year Plan
Guo Shi Jian Zheng Jian Fa [2022] No.69
National Medical Products Administration, China National Intellectual Property Administration, market supervision bureaus (departments and commissions) of all provinces, autonomous regions, municipalities directly under the Central Government and Xinjiang Production and Construction Corps, and all departments and directly affiliated units of the General Administration:
The Development Plan for Certification, Accreditation, Inspection and Testing in the 14th Five-Year Plan has been adopted at the 9th executive meeting of the General Administration of Market Supervision on July 15th, 2022, and is hereby printed and distributed to you. Please implement it carefully.
General administration of market supervision
July 29, 2022
(This piece is publicly released)
Development Plan of Certification, Accreditation, Inspection and Testing in the Tenth Five-Year Plan
In order to promote the development of certification, accreditation, inspection and testing industry during the 14th Five-Year Plan period, this plan is formulated according to the 14th Five-Year Plan for National Economic and Social Development of People’s Republic of China (PRC) and the Outline of Long-term Objectives in 2035 and the 14th Five-Year Plan for Market Supervision Modernization.
I. Compilation background
(1) Development achievements during the Thirteenth Five-Year Plan period
During the "Thirteenth Five-Year Plan" period, closely following the essential attribute of "passing on trust and serving development" in certification and accreditation inspection and testing, with reform and innovation as the driving force, we made great efforts to improve the certification and accreditation inspection and testing system, continuously strengthened the construction of industry governance capacity, comprehensively improved the supply level, and comprehensively served the economic and social development, making positive contributions to building a quality power and realizing the strategic goal of building a well-off society in an all-round way.
-the functional status has been significantly improved. The General Secretary of the Supreme Leader made an important exposition on "promoting the construction of quality certification system", which provided a fundamental basis for certification, accreditation, inspection and testing. The State Council issued the Opinions on Strengthening the Construction of Quality Certification System and Promoting Total Quality Management (Guo Fa [2018] No.3), clearly taking quality certification as an important starting point for deepening the supply-side structural reform and the "streamline administration, delegate power, strengthen regulation and improve services" reform, and making comprehensive arrangements for the construction of quality certification system. Inspection, testing and certification service industry is listed in the national strategic emerging industry classification and plays an important role in national economic and social development; Certification and accreditation inspection and testing are brought into the unified market supervision system, the responsibilities of the National Certification and Accreditation Supervision and Management Committee are assigned to the State Administration of Market Supervision and management, and the administrative supervision function is further strengthened. China’s certification and accreditation inspection and testing industry has entered a high-quality development stage.
-Deepen reform and continue to advance. The reform of compulsory product certification system has been deepened, and the catalogue of compulsory product certification has been streamlined and integrated into 17 categories and 103 products. For 19 of them, the enterprise self-declaration method has been implemented, and the designation of compulsory certification inspection institutions has been cancelled, so as to better play the role of "ensuring the bottom line of safety"; Steadily promote the reform of qualification examination and approval of certification bodies, cancel the special management measures before the entry of foreign-funded certification bodies, and implement the notification commitment and optimize the examination and approval services in the National Pilot Free Trade Zone according to the risk level; Comprehensively deepen the reform of qualification certification of inspection and testing institutions, integrate measurement certification of product quality inspection institutions and qualification certification of inspection and testing institutions, and improve the "general requirements+industry-specific requirements" model; Optimize the administrative examination and approval procedures, fully implement the "internet plus" administrative examination and approval model, and further improve the convenience of examination and approval; The market-oriented reform of inspection, testing and certification institutions has been steadily promoted, the market vitality has been effectively stimulated, and the service ability, innovation ability and market competitiveness have been significantly enhanced.
-The institutional system is more perfect. Promote the Network Security Law, Data Security Law, Cryptography Law and other laws to be written into the relevant provisions of certification, accreditation, inspection and testing, organize the revision of the Regulations on Certification and Accreditation, complete the formulation and revision of departmental regulations and normative documents such as the Measures for the Administration of Certification Institutions, the Measures for the Supervision and Administration of Accreditation Institutions and the Measures for the Supervision and Administration of Inspection and Testing Institutions, organize the clearing of administrative normative documents and technical normative documents, and continuously strengthen institutional norms; Improve the certification system for industrial products, food and agricultural products, management systems and services, build a unified certification and labeling system for green products, improve the national network security certification system, establish certification systems for rail transit equipment, robots, drones, Beidou basic products and other new fields, issue a number of accreditation systems for e-commerce, services, greenhouse gas certification and verification, scientific research laboratories, and promote the establishment and implementation of a unified qualification certification system for inspection and testing institutions in road transport, environment, forestry and other industries, and standardize and guide them.
-The effectiveness of services has become increasingly evident. The field of certification, accreditation, inspection and testing services has been continuously expanded and widely used in various fields of national economy and social development, and the role of "passing trust and serving development" has become increasingly prominent. Strictly enforce product certification management, strengthen certification, accreditation, inspection and testing in food safety, network security, public health and other fields, and effectively protect national security, social public safety and personal health and safety; Improve the certification, accreditation, inspection and testing system of key industries, build an upgraded version of quality management system certification, implement high-end quality certification, and implement the project of "same line, same standard and homogeneity" in domestic and foreign trade, effectively promoting the quality improvement of products and services; Extensively carry out certification and accreditation work in the fields of organic, energy conservation, environmental protection and low carbon, comprehensively implement unified green product certification, and strive to improve the service capabilities of motor vehicle testing, environmental monitoring, carbon emission verification, etc., and help poverty alleviation, green development and ecological civilization construction. Significant results; Strengthen the work of international cooperation and mutual recognition of conformity assessment and technical trade measures, implement the vision and action of "One Belt, One Road" construction of certification and accreditation services, and promote the obvious improvement of trade facilitation.
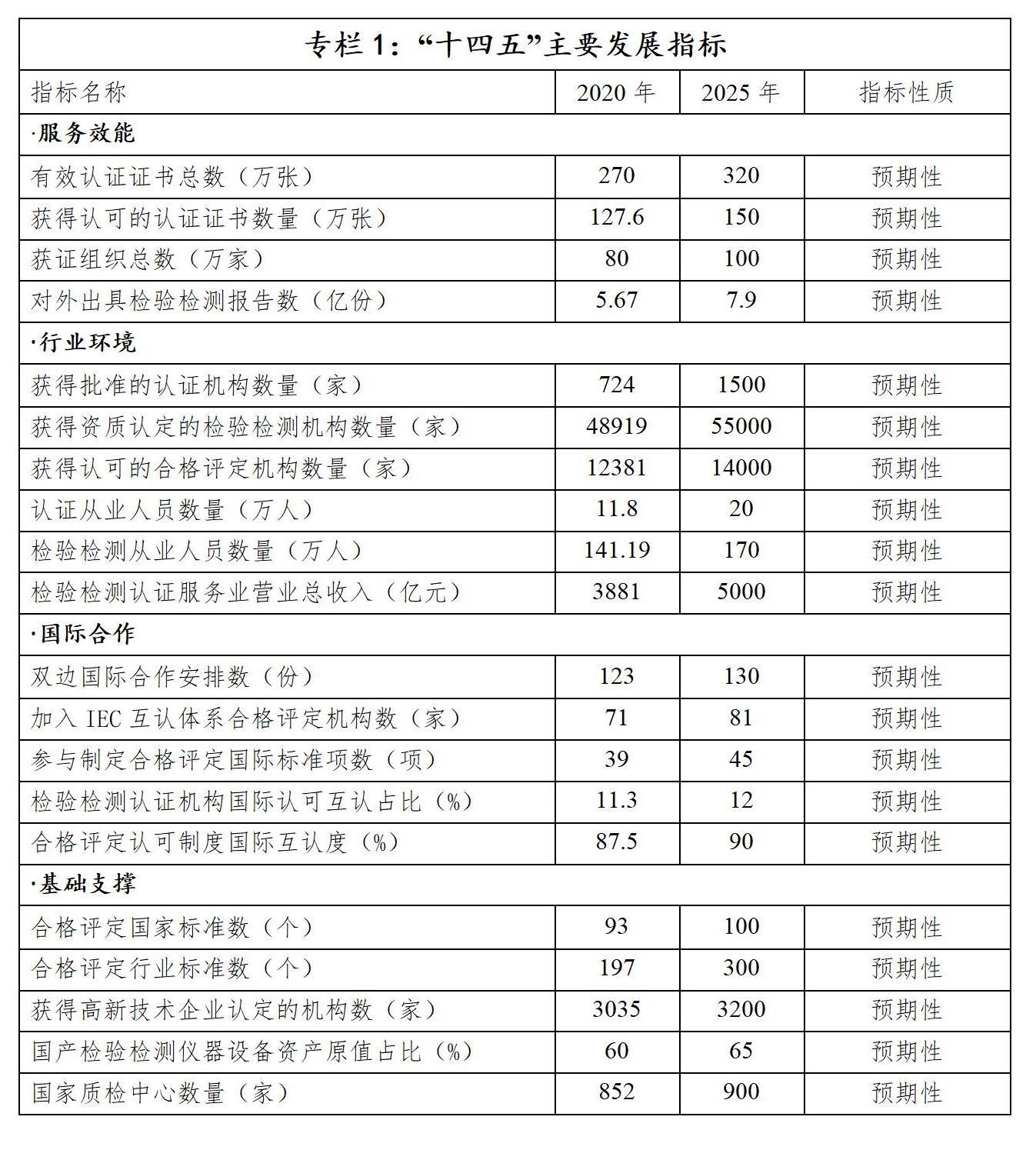
-upgrading the development of the industry. The certification and accreditation inspection and testing service industry has developed rapidly, its comprehensive strength has been significantly enhanced, and its quality and efficiency have been continuously improved. By the end of 2020, there were 724 approved certification bodies in China, with 2.7 million valid certification certificates and 800,000 certified organizations. There are 48,000 inspection and testing institutions with qualifications, and 590 million inspection and testing reports have been issued; There are 12,381 accredited conformity assessment institutions, and 1,276,000 certificates are allowed to use the accreditation marks. The output value of inspection, testing and certification service industry is 388.1 billion yuan, with an average annual growth rate of 15% during the 13th Five-Year Plan period, making it the fastest growing and most potential inspection, testing and certification service market in the world. The innovation ability, service ability and market competitiveness of institutions have improved as a whole. The number of institutions recognized by high-tech enterprises has increased by 1.5 times compared with the end of the Twelfth Five-Year Plan, and the business of institutions above designated size has reached 85%. A number of institutional brands with strong technical, management and service advantages have emerged.
-The market order is constantly standardized. Give full play to the functional advantages of unified market supervision and comprehensive administrative law enforcement system, constantly improve the five-in-one industry governance system of "legal norms, administrative supervision, recognition and restraint, industry self-discipline and social supervision", establish a new supervision mechanism with "double random and one open" supervision as the basic means, supplemented by key supervision and based on credit supervision, actively use smart supervision means such as the Internet and big data, and explore the implementation of online and offline integrated supervision modes and methods. In view of the chaos in the certification and testing industry that has been strongly reflected in the society, we organized actions such as centralized rectification of the certification and testing market and special rectification of the certification activities of epidemic prevention products, severely cracked down on illegal acts such as false certification, issuing false and false testing reports, buying and selling certificates, and selling counterfeit testing reports online, effectively safeguarding the credibility of certification and accreditation testing activities, and the order of the certification and testing market improved significantly.
-The international influence has gradually increased. China has joined 21 international organizations for conformity assessment, held a series of important positions in IEC, ISO, IAF and other international organizations, actively participated in the formulation of international standards and rules for conformity assessment, established cooperation mechanisms with more than 30 countries and regions, signed 15 multilateral mutual recognition agreements and 123 bilateral cooperation and mutual recognition arrangements, and achieved cooperation and mutual recognition results for conformity assessment under the framework of Regional Comprehensive Economic Partnership Agreement (RCEP). Substantial progress has been made in the construction of the "Belt and Road" certification and accreditation cooperation mechanism, and the cooperation between the mainland, Hong Kong and Macao in certification and accreditation inspection and testing has been deepened, and the role of certification and accreditation inspection and testing in promoting international trade facilitation has become increasingly apparent.
(B) "14" period facing the new situation and new requirements
The "Fourteenth Five-Year Plan" period is the first five years for China to start a new journey of building a socialist modern country in an all-round way and March towards the goal of the second century. China has turned to the stage of high-quality development, coordinating both domestic and international situations, coordinating development and safety, speeding up the construction of a modern economic system, speeding up the construction of a new development pattern with the domestic big cycle as the main body and the domestic and international double cycles promoting each other, and promoting the modernization of the national governance system and governance capacity, which has put forward higher requirements for certification and accreditation inspection and testing. The 14th Five-Year Plan for National Economic and Social Development and the Outline of Long-term Goals in 2035 clearly defined the objectives and tasks of the development of certification, accreditation, inspection and testing in many aspects, such as building a strong country, promoting the prosperity and development of the service industry, building a high-standard market system, promoting the dual-cycle of international and domestic development, and accelerating the development of green transformation. The CPC Central Committee and the State Council made a series of decisions and arrangements, and put forward clear requirements for certification and accreditation inspection and testing. The 14th Five-Year Plan for Modernization of Market Supervision proposes to build a modern market supervision system, improve the national quality infrastructure, and define the work measures in the fields of certification, accreditation, inspection and testing in many aspects, such as optimizing the business environment, strengthening the comprehensive management of market order, promoting market circulation, serving high-quality development, and strengthening the protection of consumers’ rights and interests. Certification, accreditation, inspection and testing are promising and face rare opportunities for development.
At the same time, there are still some problems in certification, accreditation, inspection and testing, such as inadequate supply capacity, insufficient social credibility, weak brand influence, and the need to improve international discourse power. We must make efforts to supplement shortcomings, strong and weak items, consolidate advantages and promote promotion in accordance with the requirements of basing ourselves on the new development stage, implementing new development concepts, building a new development pattern and promoting high-quality development, so as to promote certification, accreditation, inspection and testing to take the road of high-quality development and provide strong support for high-quality development of the economy and society.
Second, the overall requirements
(A) the guiding ideology
Guided by the Supreme Leader’s Socialism with Chinese characteristics Thought in the New Era, fully implementing the spirit of the 19th National Congress of the Communist Party of China and the previous plenary sessions of the 19th National Congress, building a new development pattern based on the new development stage, promoting high-quality development as the theme, deepening supply-side structural reform as the main line, meeting the people’s growing needs for a better life as the fundamental purpose, and taking reform and innovation as the fundamental driving force, establishing the concept of "big market, big quality and big supervision". Focus on the development goal of "marketization, internationalization, specialization, intensification and standardization", focus on deepening reform, strengthen system supervision, optimize service supply, give full play to the positive role of certification and accreditation inspection as a "physical examination certificate" for quality management, a "letter of credit" for market economy and a "passport" for international trade, and make new contributions to the construction of a modern market supervision system and the realization of the national development goal of the 14 th Five-Year Plan.
(2) Basic principles
1. Adhere to the party’s leadership and highlight the people-oriented.
Highlight political orientation, strengthen the Party’s leadership over certification and accreditation inspection and testing, fully implement the decision-making arrangements of the CPC Central Committee and the State Council, adhere to the people-centered development thought, take meeting the people’s needs for a better life as the fundamental purpose, take solving the people’s immediate problems as the focus of work, and practice the essential attribute of "passing trust and serving development" of certification and accreditation inspection and testing with practical actions to enhance the people’s sense of gain.
2. Adhere to the system concept and deepen reform and innovation.
Highlight the problem orientation, deepen the reform to solve the deep-seated contradictions and problems in development, comprehensively promote the "streamline administration, delegate power, strengthen regulation and improve services" reform in a wider and deeper scope, focus on promoting reforms in key areas such as market access, system implementation, supervision and management, and international cooperation, enhance the integrity, systematicness and synergy of the reform, and strive to create a market-oriented, rule-of-law and international business environment to further stimulate market vitality.
3. Adhere to market dominance and improve supply capacity.
Highlight demand orientation, guide the improvement of service supply capacity with market demand, stimulate market demand with the improvement of service supply capacity, form a higher level dynamic balance between demand traction supply and supply creation demand, expand the high-quality supply of certification and accreditation inspection and testing, and serve the high-quality development of economy and society.
4. Adhere to overall consideration and serve the overall development.
Highlight the goal orientation, focus on the economic and social development goals during the 14 th Five-Year Plan period, and comprehensively improve the ability level of certification, accreditation, inspection and testing services to build a new development pattern. Focusing on promoting domestic and international double circulation, promoting the combination of "bringing in" and "going out" of certification and accreditation inspection and testing; Focusing on overall development and safety, we should adhere to the combination of "ensuring the bottom line of safety" and "drawing high-quality lines"
5. Adhere to pluralistic governance and safeguard social public trust.
Highlight the result orientation, give full play to the comprehensive efficiency of the unified market supervision system, improve the pluralistic co-governance system combining "legal norms, administrative supervision, accreditation and restraint, industry self-discipline and social supervision", strengthen system supervision and comprehensive management, build a traceability mechanism for the whole process of certification and accreditation inspection and testing activities, and resolutely safeguard the credibility and effectiveness of certification and accreditation inspection and testing work.
(3) Development goals
Focusing on the development requirements of "marketization, internationalization, specialization, intensification and standardization", we will speed up the construction of a certification, accreditation, inspection and testing system with unified management, joint implementation, authoritative public trust and universal mutual recognition, better serve the high-quality development of the economy and society, and strive to achieve the following main objectives.
1. New progress has been made in market-oriented reform, and market vitality has been effectively stimulated. The reform in the fields of qualification examination and approval of certification institutions, qualification identification of inspection and testing institutions, Certification staff management and compulsory product certification has been deepened in an all-round way, the market-oriented allocation mechanism of inspection, testing and certification elements has been improved, the third-party attributes of certification and accreditation inspection and testing institutions have become more distinct, and the market vitality and market competitiveness have been continuously improved.
2. International development has achieved new breakthroughs and its international influence has been significantly enhanced. Follow the internationally accepted rules and constantly improve the conformity assessment system combining internationalization with China. China has made new breakthroughs in participating in the formulation of international standards and rules for conformity assessment, and its participation and voice in international conformity assessment activities have been continuously improved; Establish a normalized bilateral cooperation mechanism with major trading partners, and establish an in-depth participation mechanism with major conformity assessment international organizations. The multi-bilateral cooperation and mutual recognition arrangement covers a wider range, and the "Belt and Road" certification and recognition cooperation mechanism has achieved substantial results. The international mutual recognition of China’s certification and recognition system has improved significantly, and the international business of conformity assessment institutions has expanded significantly, forming a number of institutional brands and institutional brands with international influence.
3. Professionalization has reached a new level and service supply has been continuously optimized. The innovation, management, service ability and comprehensive strength of certification and accreditation inspection and testing industry have been improved as a whole, the ability and quality of employees and staff have been continuously optimized, the proportion of innovation R&D investment and output has been gradually increased, the certification and accreditation system in new fields and the supply of inspection and testing services have been continuously increased, the application of key technologies for conformity assessment has achieved good results, the certification and accreditation inspection and testing technology in key areas such as national security and strategic industries has been safe and controllable, the problem of "sticking the neck" of inspection and testing instruments and equipment has been effectively solved, and the digital application level in the field of conformity assessment has been gradually improved.
4. Intensive integration forms a new pattern, and the industry structure tends to be optimized. The phenomenon of "small scattered and weak" in inspection, testing and certification industry has been obviously improved, and the structural layout of inspection, testing and certification service industry is more reasonable; The comprehensive strength of large-scale institutions has been significantly enhanced, and the leading role has been effectively played; The professional ability of small and medium-sized institutions has been significantly improved, forming a number of "specialized and innovative" institutions; New progress has been made in the construction of inspection and certification public service platform and inspection and testing high-tech service industry cluster, and the radiation-driven effect is more obvious.
5. Standardized development has taken on a new look, and industry governance has been significantly strengthened. The supervision system combining "legal norms, administrative supervision, recognition and restraint, industry self-discipline and social supervision" is more complete; A new supervision mechanism based on "double random and one open" supervision is established in an all-round way, supplemented by key supervision and based on credit supervision. The "internet plus supervision" model is fully operational, forming a systematic supervision and collaborative supervision pattern with multi-sector joint supervision, integration of various supervision means and continuous innovation of supervision mechanism and methods; The effectiveness of supervision and rectification in the field of inspection, testing and certification continued to deepen, without regional, systematic and industrial risks, and the effectiveness and credibility of certification and accreditation inspection and testing continued to improve.
III. Development tasks
(A) all-round service and high-quality development
Implement the new development concept of innovation, coordination, green, openness and sharing, further promote the construction of a quality power, vigorously carry out quality improvement actions, comprehensively improve the supply level of certification and accreditation inspection and testing services, promote quality reform, power reform and efficiency reform, promote quality improvement and industrial upgrading, and boost high-quality economic and social development.
1. Building a service manufacturing power and a quality power.
(1) Promote the upgrading of industrial basic capacity. Focusing on promoting a strong industrial base, we will strengthen the supporting capacity of certification, accreditation, inspection and testing of basic raw materials, basic components, basic processes and infrastructure, and support the implementation of industrial base reconstruction projects. Promote the improvement of inspection, testing and certification capabilities in basic raw material industries such as steel, nonferrous metals, petrochemicals, light industry, textiles and building materials, support scientific research institutes, key enterprises and institutions to form inspection, testing and certification innovation alliances, strengthen key basic materials and new materials inspection and testing technology research, establish a certification system with high industry influence, and promote the adjustment and upgrading of industrial structure of basic raw materials; Improve the certification system of basic parts and components, strengthen the ability to ensure the consistency of certification, enhance the international mutual recognition of electronic component certification, promote the expansion and application of Beidou basic product certification, and promote the quality improvement of basic parts and components; Focusing on the transformation and upgrading of basic processes, we will jointly promote the optimization and improvement of conformity assessment schemes in related industries, and enhance the ability of enterprise quality diagnosis, improvement and verification; Establish and improve the certification system and inspection and testing system in the fields of Internet of Things, industrial Internet and vehicle networking, and strengthen the support and guarantee capacity of the new generation of information infrastructure construction.
(2) Promote the modernization of industrial chain supply chain. Improve the quality certification and inspection system covering the whole process of the industrial chain supply chain, and promote the safe and smooth supply chain of the industrial chain and optimize and upgrade. Using international advanced quality standards and methods, in key industries such as aviation, railway, urban rail transit, automobile, construction, medicine, information, environmental protection, etc., explore the establishment of quality certification system suitable for the characteristics of the industry, and promote the extension of quality management to the whole supply chain, the whole industrial chain and the whole product life cycle; Promote the certification of new management systems such as supply chain safety management system, business continuity management systems and compliance management system to improve the management level of supply chain; Optimize the inspection and certification service mode and industrial layout, provide integrated services for the upstream and downstream of the industrial chain supply chain, encourage the head enterprises and cluster enterprises of the industrial chain supply chain to guide the adoption of letters, reduce the market procurement cost and improve the operational efficiency of the industrial chain supply chain.
(3) Support the optimization and upgrading of manufacturing industry. We will implement the "Quality Certification Promotion Action for Key Industries", carry out the "Quality Management System Certification Promotion Activity" in depth, guide all kinds of enterprises to improve their quality management level, encourage certification institutions to develop high-end quality certification higher than the general standards of the industry, vigorously carry out the certification of rail transit, intelligent equipment, unmanned aerial vehicles, robots, smart home appliances, car networking products, explore the certification of new energy storage, Internet of Things, blockchain and privacy computing, and promote the upgrading of manufacturing industry. Implement "inspection and testing to promote industrial upgrading", improve the technical support capacity of inspection and testing in key industries, build a professional inspection and testing platform based on the layout of strategic emerging industries and manufacturing industrial clusters, break through a number of basic, public welfare and industrial common technical bottlenecks, and support enterprise innovation and development and industrial transformation and upgrading.
(4) Helping the high-quality development of the service industry. Accelerate the construction of service industry certification and accreditation system, carry out service certification demonstration activities, promote the standardization and branding of service industry, and improve service quality and efficiency. In the field of producer services, actively promote the service certification of engineering construction, digital design, logistics and transportation, energy conservation and environmental protection services, green data center, strengthen the inspection and testing capacity building of major transportation infrastructure, urban rail transit operation and maintenance, postal express packaging, intelligent logistics equipment, promote the development of new formats such as service-oriented manufacturing, and promote the producer services to extend to specialization and high-end value chain; In the field of life service industry, we will extensively carry out service certification for health, education, sports, pension, finance, after-sales goods and e-commerce, improve the quality of life service, and promote the life service industry to upgrade to high quality and diversification.
2. Smooth domestic and international double circulation.
(1) to promote the construction of a unified national market. Focusing on the construction of an efficient, standardized, fair competition and fully open national unified market, we will accelerate the construction of a certification, accreditation, inspection and testing system with unified management, joint implementation, authoritative public trust and universal mutual recognition, improve the market credit mechanism, promote the elimination of regional market barriers, and promote the market-oriented allocation of factor resources. Implement the decision-making arrangements of the CPC Central Committee and the State Council on the whole process reform of market access and circulation management in automobile, electronic and electrical industries, promote the reform of industry access system, cancel repeated evaluation projects, and reduce market transaction costs; Clean up the administrative licensing and industry evaluation system involving certification, accreditation, inspection and testing, promote the social-oriented third-party technology evaluation activities to follow general norms and standards, and gradually change to a unified national certification system. In areas where a unified national certification system has been established, similar conformity assessment projects will no longer be established.
(2) Promote coordinated regional development. Encourage local governments to introduce policies and measures to strengthen certification, accreditation, inspection and testing, guide the establishment of regional linkage mechanisms, promote the sharing of certification, accreditation, inspection and testing resources, platform sharing, mutual recognition of results, supervision and mutual assistance, support the integrated development of inspection, testing and certification services in urban agglomerations such as the Yangtze River Delta, the Pearl River Delta, Beijing-Tianjin-Hebei and Chengdu-Chongqing, and promote the circulation of regional factors and the smooth market; Implement CEPA agreement and Guangdong-Hong Kong-Macao Greater Bay Area’s development strategy, promote the in-depth cooperation of inspection, testing and certification services in Greater Bay Area, explore "Bay Area Certification", build a high-end certification brand with international influence, and realize "one certification, three places pass"; Implement certification, accreditation, inspection and testing measures to aid Tibet and Xinjiang, support the revitalization and development of old areas, and support the certification of organic products, cotton and other products and the construction of national quality inspection centers; Standardize and guide all localities to use certification to cultivate regional quality brands and enhance regional economic competitiveness.
(3) Promote the integrated development of domestic and foreign trade. Promote the convergence of domestic and foreign trade certification and accreditation, and promote the improvement of the integrated control system of domestic and foreign trade. Encourage the international development of third-party testing and certification institutions and provide integrated services for conformity assessment for domestic and foreign trade. We will improve the promotion and implementation mechanism of "the same line, the same standard and the same homogeneity" (hereinafter referred to as "the same quality"), which is integrated inside and outside, connected from top to bottom, coordinated by departments and connected with supply and demand, support enterprises to develop "the same quality" products, expand the application scope of "the same quality" products to general consumer goods and industrial products, and accurately meet consumer demand. Improve the public information service platform of "Three Tong" products, encourage certification institutions to carry out quality evaluation and technical assistance, and maintain the high-end quality brand reputation of "Three Tong" products. Strengthen cross-border e-commerce quality certification and inspection and testing technical services, and improve the quality and safety level of imported goods and goods exported to domestic sales.
3. Help rural revitalization
(1) Innovative conformity assessment service rural revitalization work mode. Strengthen the research on the path of quality certification, inspection and testing to serve rural revitalization, carry out the strategic work of quality certification to serve rural revitalization, explore the linkage mode of "high-standard market+high-quality production area" by means of quality certification, benchmark the demand of high-end market, cultivate regional high-end quality brands such as "Shenzhen Products" and "Lishui Mountain Farming" through certification and evaluation, improve the docking mechanism of production and marketing of high-quality agricultural and sideline products, and promote the high-quality development of rural revitalization. Encourage quality certification and inspection services to the countryside, provide accurate services for agricultural industrial parks, agricultural production and operation enterprises, agricultural mutual cooperatives and farmers, and strengthen technical support for rural revitalization.
(2) Promote the quality of agricultural products supply. Strengthen the supply of quality certification and inspection and testing services, and improve the certification and inspection and testing system of food and agricultural products in the whole process from field to table. Encourage agricultural products production and marketing enterprises and farmers’ professional cooperatives to carry out green food, organic products, hazard analysis and critical control points (HACCP), good agricultural practices (GAP) and other food and agricultural products certification, improve the quality and added value of agricultural products, and support the construction of green agricultural products bases, ecological farms and modern agricultural industrial parks; Strengthen the capacity building of city and county agricultural products inspection and testing institutions, focusing on improving the detection capabilities of food safety and livestock diseases.
(3) Promote the development of rural industries. Build a quality certification and inspection system covering production, processing, warehousing, transportation, distribution, preservation and other links to serve the modernization of rural industries. In view of the quality, flavor, green and other attributes, we will implement the certification mode of "product+service", actively carry out the certification of special agricultural products such as organic products and selenium-enriched products, and the certification of rural tourism, leisure agriculture, homestay services, farming culture experience and healthy old-age care, strengthen the support of inspection and testing services in agriculture and rural areas, focus on strengthening the construction of inspection and testing capabilities such as seeds, agricultural materials, agricultural machinery and cold chain logistics, and support the development of agricultural technology services, cold chain logistics and chain distribution in rural areas.
(4) Helping the construction of beautiful countryside. Implement the concept of "Lucid waters and lush mountains are invaluable assets", improve the quality certification and inspection technical services in rural infrastructure, public services, human settlements and other fields, and explore the establishment of a third-party evaluation system in beautiful countryside. Strengthen the construction of inspection and testing capabilities of soil, air and water environment, serve the prevention and control of agricultural non-point source pollution, and optimize the livable environment in rural areas.
4. Support green transformation and development
(1) Increase the supply of green products. Accelerate the implementation of a unified green product certification and labeling system, promote the integration of "green-related" evaluation systems in an orderly manner, and include more products with great ecological impact, strong consumer demand, strong industrial relevance, high social concern and large international trade volume into the green product certification catalogue, and explore the requirements for the control of new pollutants into the green product certification system. Improve the promotion mechanism of green product certification, promote the improvement of government green procurement system, encourage society to give priority to purchasing certified green products and increase the supply of green products. Accelerate the innovative research and development of green product evaluation technology, cultivate a number of professional service institutions for green products, and improve the technical support system for green products.
(2) Help fight against pollution. Focus on the field of high emission and high pollution, study and carry out cleaner production evaluation and certification, and effectively standardize the certification activities of products related to environmental protection treatment facilities and equipment; Deepen the special management of road transport safety, strictly enforce the certification requirements for motor vehicle emissions and other projects, and crack down on illegal activities in the field of compulsory product certification for illegally modifying road transport vehicles; Improve the accreditation system of ecological environment monitoring institutions, improve the "double random" spot check mechanism of ecological environment monitoring institutions, establish the directory database of ecological environment monitoring institutions and the directory database of inspectors, and promote the healthy development of ecological environment monitoring; Carry out joint supervision and law enforcement by departments, and severely crack down on illegal and criminal acts such as falsification of detection data by ecological environment monitoring agencies and motor vehicle emission inspection agencies.
(3) Serving the goal of "double carbon". Accelerate the construction of a conformity assessment system in the carbon field, focus on industries such as electric power, chemical industry, building materials, steel, nonferrous metals, paper making, automobile, etc., study and formulate conformity assessment solutions in the whole process and life cycle, strengthen the capacity building of carbon emission conformity assessment, improve the accreditation system of carbon emission certification and verification institutions, and promote the coordinated application and innovative development of various conformity assessment tools in the carbon field, such as products, processes, systems, service certification and validation verification, inspection and testing. Improve the carbon sequestration certification system of ecosystems such as forest certification, and standardize the certification services such as carbon footprint and carbon labeling; Improve the relevant standards for greenhouse gas emission verification, strengthen key technical research on carbon verification, certification and accreditation, strengthen the management of greenhouse gas voluntary emission reduction verification and verification institutions and activities, and accelerate the construction of carbon emission verification and testing technology laboratories.
(4) Improve the conformity assessment system in the field of energy and natural resources. Vigorously promote the safety certification and performance certification of equipment such as wind power, photovoltaic power generation, biomass energy, nuclear power, ocean energy, etc., improve the new energy certification system, carry out the product certification of step-by-step utilization of power batteries in new energy vehicles, improve the technical level of residual energy detection of power batteries, strengthen the inspection and testing capacity building in the fields of fuel, natural gas, hydrogen energy, charging piles and new energy storage facilities, and promote the safe and efficient utilization of energy and transformation and development; Promote the application of certification, accreditation, inspection and testing in the field of natural resources, improve the technical specifications for certification, evaluation, inspection and testing of natural resources such as forests and grasses, land, oceans, geology and minerals, improve the certification system for sustainable management of forests and grasses, expand the technical support capabilities of natural resources investigation and monitoring, satellite remote sensing and so on, and promote the protection and intensive utilization of natural resources.
5. Serve the construction of "Safe China" and "Healthy China"
(1) Strengthen product quality and safety guarantee. Give full play to the role of compulsory product certification to ensure the bottom line of safety, implement compulsory certification (CCC certification) for products related to personal health, safety and environmental protection, and implement dynamic management of certified product catalogue according to risk level; Strengthen the construction of product quality and safety inspection and testing capabilities, focus on consolidating inspection and testing technical institutions in cities and counties, local areas, western regions and industrial clusters, and effectively support market supervision and administrative law enforcement such as product quality supervision and spot checks; Focus on the supervision of CCC certification, motor vehicle inspection, ecological environment monitoring, medical device inspection and other high-risk areas, strengthen supervision and inspection, and severely crack down on illegal acts such as leaving the factory, selling, importing or using products in CCC catalogue in other business activities without certification, forging and fraudulently using certification marks, false certification, unqualified testing and issuing false testing reports; Carry out research on the public service mechanism of inspection, testing and certification to provide effective technical support for safeguarding consumers’ rights and interests.
(2) Strengthen food safety. Implement the "four strictest" requirements, build a certification and inspection system for food and agricultural products from farmland to table, and improve the ability of food safety. Encourage food production and operation enterprises to obtain certification, strengthen the certification evaluation and post-certification supervision of food production and operation enterprises that have passed the certification of good production practices, hazard analysis and critical control point management system, and continuously improve the level of food safety management. Coordinate the promotion of national food inspection and testing capacity building and strengthen the supervision of food inspection and testing institutions.
(3) Strengthen network security. Improve the network information security certification and accreditation inspection and testing system, establish and improve the network security certification and accreditation system covering information technology products, systems, services, management systems and personnel, strengthen the construction of inspection and testing capabilities, enhance the network security guarantee capability, and serve the network power and the construction of digital China. Carry out personal information protection certification, mobile Internet application (APP) security certification, data security management certification, commercial password product certification, financial technology product certification, etc., and strengthen personal information and data security protection; Strengthen the technical capacity building of network security certification, promote the implementation of information security assurance practitioners certification, and improve the qualification evaluation norms of network security inspection and testing institutions; Improve the network information security certification and acceptance mechanism, promote cross-industry sharing and acceptance of certification results, and promote the healthy and orderly development of the network information industry and market system.
(4) Strengthen health service guarantee. Accelerate the construction of a certification and accreditation inspection and testing system in the field of public health, and improve the supply of public health services. Standardize the management system certification of medical institutions and improve the service quality of medical institutions; Promote the quality certification of epidemic prevention products, strengthen the construction of barrier-free environment quality certification system, actively carry out the certification of health services and old-age services, and help the health of the whole people and the elderly and the disabled; Accelerate the construction of Chinese medicine testing and certification system, study and establish independent innovation certification systems for authentic Chinese herbal medicines and Chinese medicine drinks, and promote the high-quality development of Chinese medicine; We will improve the emergency response mechanism for inspection and testing of major public health emergencies, open an "emergency channel" for inspection and testing capacity licensing for major emergency needs such as epidemic prevention and control, strengthen the capacity verification of relevant inspection and testing institutions, and improve the inspection and testing support capabilities.

(B) accelerate the realization of stronger and better industries.
In accordance with the requirements of high-quality development, strengthen the construction of certification and accreditation inspection and testing industry, improve the development environment, optimize the layout of the industry, improve the service capacity, service level and service quality of the industry, and establish a certification and accreditation inspection and testing service system that is closely linked and integrated with the modern industrial system.
1. Optimize the industry development environment
(1) Deepen the reform of market access system. We will comprehensively deepen the reform of the market access system for certification and testing, such as the qualification examination and approval of certification bodies, the designation of compulsory product certification implementation agencies, and the qualification identification of inspection and testing institutions, so as to stimulate market vitality. Fully implement the reform of qualification examination and approval of certification bodies, respectively implement classified examination and approval combining informing commitment and optimizing examination and approval services according to the degree of risk, and improve the verification mechanism of continuous conformity of certification bodies’ qualifications; Improve the way to designate institutions for compulsory product certification, expand the number of designated institutions for compulsory product certification, and accelerate the formation of a dynamic balance between the supply of designated institutions and market demand; We will fully implement the notification and commitment system for qualification identification of inspection and testing institutions, further reduce the time limit for qualification identification and evaluation, and streamline and optimize the procedures and contents of licensing and evaluation. Improve the online examination and approval system for the qualification examination and approval of certification bodies and the qualification identification of inspection and testing institutions, fully implement online processing, and improve the convenience of examination and approval.
(2) Cultivate and expand all kinds of practitioners. We will build a unified, open, competitive and dynamic market system for inspection, testing and certification, and create a market environment in which all types of ownership market players are treated equally and compete fairly. Adhere to the impartial and independent third-party attribute, actively promote the market-oriented reform of inspection, testing and certification institutions, establish and improve the business management model to adapt to market competition, and stimulate market vitality; Support private institutions to become stronger and bigger, encourage non-public capital to participate in the restructuring, capital increase and share expansion and management of state-owned institutions, and enhance their competitiveness; Encourage foreign-funded institutions to enter the domestic inspection, testing and certification market, actively introduce foreign advanced conformity assessment standards, technologies and services, and fully implement national treatment.
(3) Improve the management system of certified practitioners. Cancel the compulsory registration requirements for Certification staff’s professional qualifications, and establish a certification system for certified practitioners. We will build a capacity evaluation and credit evaluation system in Certification staff, formulate scientific and reasonable technical capacity requirements and professional ethics, promote the continuous improvement of capacity and quality, and form a supply mechanism for certified practitioners to meet the needs of high-quality development of the industry. Strengthen the administrative supervision and accreditation supervision of certification bodies to carry out Certification staff capacity evaluation and use management activities, and implement the main responsibility of certification bodies to evaluate and manage Certification staff.
(4) Improve the acceptance mechanism of certification, accreditation, inspection and testing. Promote national laws and regulations, departmental rules and industrial policies to establish a credit acceptance system, improve the credit acceptance mechanism at various levels such as government, industry and society, and promote the wide acceptance of credit certification and accreditation inspection results in the fields of market procurement, industry management, administrative supervision and social governance. Promote fair acceptance of trust, break departmental monopoly and industry barriers, and realize mutual recognition and universality of certification and accreditation inspection results.
(5) Strengthen policy guidance and industry services. Fully implement the decision-making arrangements of the CPC Central Committee and the State Council on promoting the development of certification, accreditation, inspection and testing, promote the introduction of specific policies and measures by various localities and departments, study and formulate detailed implementation measures of national industrial development policies in the field of certification, accreditation, inspection and testing, and increase policy guidance and support; Strengthen fair competition review, promote the elimination of discriminatory requirements involving certification and accreditation inspection and testing in bidding, government procurement, government procurement of services and other fields, and break departmental monopoly and industry barriers; Improve the statistical investigation system of certification, accreditation, inspection and testing, carry out research on the measurement indicators of high-quality development of the industry, standardize the release of statistical data and evaluation indicators, and enhance the guiding function of industry development; Strengthen the service function of trade associations and enhance the functions of industry coordination, rights protection, technical exchange and personnel training.
2. Improve professional service capabilities
(1) Promote the integrated development of quality certification and inspection. Encourage practitioners to expand the business field of inspection, testing and certification through business cooperation, asset reorganization and mergers and acquisitions, make up for the shortcomings of qualification ability, extend the service chain of conformity assessment, and form a compound and integrated conformity assessment ability; Encourage institutions to develop integrated conformity assessment solutions according to users’ needs and improve their comprehensive service capabilities; Encourage certification bodies to expand diversified certification services such as products, systems and services with strong relevance under the premise of having corresponding capabilities; Encourage inspection and testing institutions with strong professional ability and good market reputation to apply for certification qualification in the same field; Encourage inspection and certification public service platform and inspection and certification alliance to play an integrated role, optimize resource allocation, and carry out collaborative services.
(2) Improve the professional ability and brand image of inspection and certification institutions. Strengthen policy incentives and guidance, promote qualified inspection, testing and certification institutions to be recognized as high-tech enterprises, improve the policies and mechanisms for brand cultivation, development, incentives and protection of inspection, testing and certification industries, create a good brand growth environment, and enhance the brand image of inspection, testing and certification industries; Encourage inspection, testing and certification institutions to enhance their professional ability and enhance the trust of all parties through the recognition of recognized institutions; Support large-scale institutions to develop comprehensive integrated services, implement the industry "leader" plan, cultivate a number of industry-leading enterprises, and build internationally renowned brands; Support small and medium-sized institutions to take the development path of "specialization and innovation", cultivate a number of "individual champions" and "invisible champions", enhance their professional service capabilities, and form a distinctive brand advantage.
(3) Optimize the layout of national quality inspection centers. Strengthen the planning, construction and management of national quality inspection centers, improve the exit mechanism, and give priority to supporting the construction of a number of national quality inspection centers that serve the development of high-tech industries and strategic emerging industries; Support the National Quality Inspection Center to participate in the construction of State Key Laboratories, National Manufacturing Innovation Center, National Industrial Technology Innovation Center and National Technology Innovation Center, undertake the first set of major technical equipment testing and evaluation, instrument and equipment development, capacity verification lead organization and other work, and give full play to the technical leading and supporting role of the National Quality Inspection Center.
(4) Promote the integrated development of national quality infrastructure. We will build a national quality infrastructure integration development system that is coordinated, coordinated, efficient and systematic, promote the coordinated interaction, collaborative innovation and integrated development of certification and accreditation inspection and measurement, standards (including standard samples) and quality management, actively carry out the construction of quality infrastructure integration service bases, support quality infrastructure service platforms and institutions to provide conformity assessment services, and strengthen the authoritative evaluation role of accreditation in the quality infrastructure system. Strengthen the positive role of quality certification, inspection and testing to promote the wide application and continuous improvement of other quality infrastructure elements, and promote the interconnection and high-quality development of national quality infrastructure.
3. Promote the digital development of conformity assessment.
(1) Innovative digital evaluation model. Organize the research and application of digital evaluation mode of conformity assessment, and use modern information technologies such as big data, blockchain and artificial intelligence to promote the digital application of conformity assessment. Carry out research and application of digital authentication mode and its key technologies based on industrial Internet and intelligent manufacturing, establish and improve digital evaluation rules and technical specifications in the field of quality authentication, and gradually popularize digital certificates; Explore the whole digital mode of inspection and testing, and promote the digital and intelligent application of inspection and testing based on digital equipment; Promote the digital evaluation of conformity assessment to extend to the whole process of industrial chain supply chain and the whole product life cycle, and enhance the digital evaluation ability.
(2) Promote the digital development of the industry. Adapt to the requirements of digital development of industry, promote the digital management of certification and accreditation inspection and testing industry, improve the digital infrastructure of certification and accreditation inspection and testing, promote the development of digital technologies such as certification and testing professional management software, intelligent testing equipment and data application terminals, and comprehensively improve the management level and develop quality and efficiency of the industry through digital technology empowerment.


(3) Vigorously improve the ability of industry governance
We will build a multi-co-governance pattern of "legal norms, administrative supervision, accreditation constraints, industry self-discipline and social supervision", improve a new supervision mechanism based on "double random and one open" supervision, supplemented by key supervision, and based on smart supervision, comprehensively strengthen the supervision capacity building of certification, accreditation, inspection and testing, and promote the standardized and orderly market of certification, accreditation, inspection and testing and the long-term healthy development of the industry.
1. Improve the supervision system of combining things before and after.
(1) Strengthen the supervision of qualification compliance of certification bodies. In order to meet the new requirements of the reform of qualification examination and approval of certification bodies, while relaxing access restrictions and improving the efficiency of examination and approval, we should improve the verification mechanism of qualification compliance of certification bodies, focus on strengthening the verification of institutions that have obtained qualifications by means of notification commitment and newly approved institutions, revoke qualifications in time for institutions whose qualification ability cannot meet the requirements continuously, and strengthen risk warning and credit punishment for institutions that operate abnormally. Improve the management system for overseas institutions to carry out conformity assessment activities in China.
(2) Implement a supervision system combining daily and special supervision. Carry out daily supervision and inspection on the inspection, testing and certification activities and results of the institutions by means of on-site inspection, file inspection, certified organization inspection and spot check of certified products. Strengthen the research and judgment of quality accidents, complaints and reports, risk monitoring, etc., and carry out targeted special supervision and inspection in a timely manner. Publicize the results of supervision and inspection according to law, and investigate the legal responsibilities of illegal institutions and employees. Improve the quality analysis and risk early warning mechanism of certified products involved in the spot check of certification effectiveness and national supervision and spot check, urge certification institutions to take post-processing measures such as revocation of problematic products and suspension of certification certificates, and force certified enterprises to strengthen quality traceability.
(3) Improve the ability verification system of inspection and testing institutions. Timely discover the problems existing in the management and technology of relevant inspection and testing institutions and urge them to rectify, so as to continuously improve the technical ability and personnel level of inspection and testing institutions in China. Improve the database and platform construction of inspection and testing institutions’ capacity verification, conduct in-depth statistical analysis on the data and results of capacity verification, and put forward white papers and capacity improvement routes for inspection and testing institutions in various fields such as electronic appliances, food environment, building materials and textiles, and bulk raw materials.
2. Improve the comprehensive supervision mechanism of multi-party cooperation.
(1) Strengthen collaborative supervision. Establish and improve the inter-departmental and inter-regional law enforcement linkage response and cooperation mechanism to realize the exchange of regulatory information, the interconnection of illegal clues and the mutual assistance of law enforcement actions. Promote the relevant administrative departments to carry out joint supervision and joint punishment in the fields of compulsory certification product access management, environmental monitoring and motor vehicle security inspection; Give full play to the comprehensive law enforcement efficiency of market supervision, strengthen the coordination of certification and testing supervision and credit supervision, law enforcement inspection, anti-monopoly law enforcement, anti-unfair competition, network supervision and advertising supervision, and organize the market supervision system to jointly handle global and cross-regional case clues; Smooth the connection between disciplines, improve the working mechanisms such as information communication, clue transfer, measures coordination and achievement sharing, and hand over the clues of discipline-related cases to the discipline inspection and supervision departments according to regulations.
(2) Strengthen the recognition constraints. Optimize the top-level design of accreditation system, better adapt to the new development trend of conformity assessment activities in various industries, and provide more ability verification services to meet the needs of conformity assessment institutions and related parties. Focus on major national strategies, and strive to improve the level of recognized technical support in the fields of energy conservation and emission reduction, food safety, network security and safe production. Strengthen the special supervision of accreditation based on risk assessment, and constantly strengthen the role of accreditation and restraint. Give full play to the recognized advantages of international mutual recognition, and promote the capacity building of inspection and certification and the acceptance of results.
(3) Strengthen industry self-discipline. Guide practitioners and practitioners to consciously abide by the "Regulations on Certification and Accreditation" and the basic norms of conformity assessment, and encourage industry self-discipline in the form of peer review. Strengthen the self-discipline management function of trade associations, establish and improve the self-discipline behavior norms and professional ethics standards of institutions, explore the establishment of systems such as "whistleblower" and internal whistleblower, encourage and guide peer supervision, standardize the behavior of employees and personnel, and safeguard the reputation of certification, accreditation, inspection and testing industries.
(4) Strengthen social supervision. We will improve the information sharing platforms such as the national public service platform for certification and accreditation information, the information inquiry system for qualification identification of inspection and testing institutions, and the inquiry system for inspection and testing report numbers, unblock the channels for reporting complaints, strengthen the investigation and evaluation of professional conduct, encourage the public to conduct information inquiry and behavior supervision on employees, attach importance to giving play to the role of news public opinion supervision, encourage the news media and online platforms to expose problem clues and illegal cases, and enhance the synergistic effect of public opinion supervision and administrative law enforcement.
3. Improve the system supervision mode with multiple measures.
(1) Full implementation of "double random and one open" supervision. First, adhere to the combination of "double random and one open" supervision and classified management. Continuously optimize and improve the directory database of employees and the directory database of supervisors. Through big data analysis, scientific classification is implemented for institutions with different risk levels and credit levels. When randomly selecting inspection objects, take differentiated supervision measures for different types of institutions and set a reasonable sampling ratio. Intensify spot checks on institutions with high risk level and low credit level. The second is to adhere to the combination of unified deployment and hierarchical implementation. The General Administration has strengthened the overall coordination of the overall inspection work, uniformly extracted the inspected objects, uniformly formulated inspection work instructions and inspection forms, unified inspection standards, and improved the standardization of inspection work. Give full play to the hierarchical management efficiency of the General Administration and local market supervision departments, and the inspection tasks shall be implemented by the General Administration and local market supervision departments at different levels to form a joint force of supervision. The third is to innovate regulatory means and strengthen the application of big data. Make full use of information technology and multi-dimensional big data analysis to find the entry point of inspection, improve the targeted accuracy of supervision and improve the efficiency of supervision.
(2) Strengthen risk monitoring and early warning. Improve the whole process traceability, risk monitoring and early warning mechanism of certification and accreditation inspection and testing activities, and organize the collection, analysis, judgment, risk monitoring and early warning, risk disposal and risk tracing of certification and accreditation inspection and testing. Carry out research on risk technical index system and improve risk monitoring index system; Improve quality analysis and public opinion monitoring mechanism, expand risk information collection channels, and strengthen risk judgment and information sharing; Improve the emergency response plan and do a good job in risk emergency response and post-treatment; Strengthen the construction of industry monitoring big data platform and carry out special monitoring and analysis in key areas; Improve the "two single maps" of integrity risk prevention and control, and strengthen the integrity risk prevention and control of qualification licensing, technical review and administrative supervision.
(3) Strengthen supervision and punishment. Implement the "who issues the certificate, who is responsible, who signs it and who is responsible", establish a system in which witnesses take overall responsibility for the inspection, testing and certification results, and strictly implement the main responsibility of the practitioners for the inspection, testing and certification results. Compaction institutions undertake the performance responsibilities entrusted by government departments, and seriously investigate and deal with the data, inaccurate results and fraudulent behaviors of relevant institutions and personnel in undertaking tasks such as product quality supervision and spot check and risk monitoring.
(4) Improve credit supervision. Strengthen the collection and sharing of credit information, establish a working mechanism connected with credit supervision information platforms such as enterprise credit information publicity platform, abnormal business list and serious illegal and untrustworthy list, and establish credit files of institutions and employees; Strictly implement the punishment for dishonesty, study and establish a joint punishment mechanism for serious illegal and untrustworthy subjects in the field of inspection, testing and certification, and implement joint punishment for inspection, testing and certification institutions, employees and certified organizations with serious dishonesty according to law to increase the cost of illegal and untrustworthy; Establish a credit repair system, and remove those who meet the repair conditions from the list of dishonesty in time according to regulations; Improve the industry credit evaluation mechanism, explore the credit rating and third-party evaluation of inspection, testing and certification industries, and promote the formation of a long-term mechanism of "disciplinary punishment for dishonesty and trustworthy incentives".
(5) Innovative and intelligent supervision. Build a smart supervision platform covering the whole process of certification, accreditation, inspection and testing activities, strengthen the construction of big data centers and information management systems, and realize real-time collection, accurate analysis and in-depth application of data information. Build an information sharing platform for the whole process of quality certification, collect the information of certification activities in real time, and break through the information bottleneck of certification implementation and supervision; Improve the network sign-in supervision system of certification on-site audit, and realize the integration of online and offline certification supervision; Relying on national inspection and testing institutions, build a number of inspection and testing data centers in key industries; Improve the information sharing and online verification mechanism, and increase the online verification of certification, accreditation, inspection and testing information docking e-commerce platforms and regulatory authorities.

(4) Effectively improve the level of international cooperation.
Grasp the new situation of opening to the outside world, deeply participate in global quality governance, promote the international development of certification and accreditation inspection and testing industry, enhance the international influence and voice of China’s certification and accreditation inspection and testing, and play a more active role in building a new pattern of international cooperation and opening up and promoting high-level opening up.
1. Deepen international cooperation and mutual recognition in all directions.
(1) Actively promote the construction of international conformity assessment organizations. Expand China’s participation and influence in international conformity assessment organizations, do a good job in recommending posts by international organizations, and promote more qualified international talents in China to hold management and technical positions; Strengthen cooperation with WTO, UNIDO, INetQI and other international organizations, promote international exchange of conformity assessment from a wider field and scope, and promote the interconnection of international quality infrastructure; Strengthen the construction of domestic counterpart working groups for conformity assessment of international organizations such as ISO, IEC, IAF and ILAC, improve the domestic operation mechanism of IEC conformity assessment system, form an alliance of domestic operation mechanism of IEC conformity assessment system, and enhance the participation of domestic enterprises and practitioners.
(2) Actively participate in the formulation of international conformity assessment standards and rules. Aiming at international capacity cooperation and the forefront of science and technology industry, we will actively put forward conformity assessment standards and rules in low-carbon and new energy fields such as offshore wind power, photovoltaic power generation, charging piles for electric vehicles, hydrogen energy and ultra-high voltage transmission, actively undertake international peer review, capacity comparison among international laboratories and other cooperation projects, actively publicize China’s excellent practice of conformity assessment through multi-bilateral international arena, promote China’s conformity assessment standards and systems to be internationally adopted, build an internationally renowned certification system brand, and strengthen its contribution to the development of international conformity assessment system.
(3) Expand and deepen the mutual recognition results of multi-bilateral cooperation. Strengthen multi-level cooperation between governments and institutions, and expand cooperation areas, partners and channels. Actively promote the expansion and upgrading of cooperation arrangements for conformity assessment under free trade zones (FTAs), solidly promote mutual recognition arrangements for conformity assessment under Regional Comprehensive Economic Partnership Agreement (RCEP), closely follow the negotiation processes such as Comprehensive and Progressive Trans-Pacific Partnership Agreement (CPTPP) and Digital Economy Partnership Agreement (DEPA), and study and propose cooperation plans in the field of conformity assessment; Accelerate the process of mutual recognition of food and agricultural products, consumer goods, equipment manufacturing, service industry and other fields, promote new breakthroughs in international mutual recognition in key areas such as information technology, high-end equipment, green products, renewable energy, and achieve a number of "small but beautiful" cooperation results in order to solve the specific needs of industries and enhance the competitiveness of related industries in the international market.
(4) Pragmatically promote the "Belt and Road" certification, accreditation, inspection and testing cooperation. Implement the "Belt and Road" cooperation initiative, focus on deepening multi-level cooperation between governments and people in the field of conformity assessment, and extensively promote cooperation in various fields such as policy coordination, technical exchange, personnel training, institutional cooperation, market opening and mutual recognition arrangements. Formulate the "One Belt, One Road" cooperation action plan for certification, accreditation, inspection and testing, organize and implement a number of effective cooperation projects such as the training of "One Belt, One Road" conformity assessment officials, technical assistance for certification and accreditation, and laboratory capacity verification, and promote the multi-bilateral mutual recognition arrangements in the fields of food and agricultural products, energy, transportation, electronic appliances, communication facilities, etc., and enhance the level of regional trade and investment facilitation.
2. Improve the facilitation level of conformity assessment service trade.
(1) Improve conformity assessment and promote international trade service system. Build a "information service platform for trade facilitation of conformity assessment services", strengthen the functions of publishing, sharing and feedback of conformity assessment information, and improve the accuracy and convenience for enterprises to obtain international conformity assessment services; Carry out in-depth analysis of the impact of conformity assessment on the trading industry, implement the project of "dynamic tracking and application of international certification and accreditation laws, regulations and standards", carry out comparative research on laws and regulations in the field of international conformity assessment, establish a database of conformity assessment information for major trading objects and trading products, track and analyze the development trend of international conformity assessment and the demand of trade industry development for conformity assessment, and study and formulate solutions for conformity assessment to promote trade facilitation under the new development pattern; Do a good job in WTO/TBT notification, consultation and evaluation, actively reflect the demands of the industry, put forward reasonable proposals, promote the fairness and transparency of conformity assessment measures in relevant countries, prevent the abuse of technical trade measures, and promote the liberalization and facilitation of trade and investment. Strengthen the assessment and response of the impact of overseas conformity assessment measures on China’s industry and trade, accelerate the pace of independent innovation and cooperation and mutual recognition of conformity assessment in key core areas, promote the stability and smoothness of the supply chain of international industrial chain, and safeguard national sovereignty and development interests.
(2) Promote the international development of professional institutions. Encourage and guide practitioners to participate in multi-bilateral cooperation and mutual recognition of conformity assessment, promote the continuous growth of the number of domestic institutions joining multilateral mutual recognition systems such as IEC and IQNet, encourage domestic institutions to set up branches abroad, support domestic and foreign institutions to carry out business cooperation, and promote the close connection between inspection, testing and certification and overseas investment and capacity cooperation projects to provide enterprises with international, localized and integrated services; Encourage practitioners to participate in the formulation of international conformity assessment standards and rules and technical exchanges, participate in international capacity cooperation and service outsourcing projects, enhance independent innovation capability and international market competitiveness, cultivate new advantages in technology, management and service, and build an internationally renowned brand of conformity assessment institutions; Accelerate the training and export of international talents from professional institutions, and cultivate a team of high-quality professional talents with international vision.

(E) efforts to consolidate the foundation support system.
Taking reform and innovation as the driving force and improving the quality of supply as the main line, we will solidly promote the construction of the rule of law, systems, science and technology, and talent teams, comprehensively strengthen basic support capabilities, and promote the high-quality development of certification and accreditation inspection and testing.
1. Strengthen the protection of law and discipline
(1) Improve the system of laws and regulations. Accelerate the revision of the "Regulations on Certification and Accreditation", study and draft the "Regulations on Inspection and Testing", promote relevant laws and regulations to improve the provisions related to certification and accreditation inspection and testing, and accelerate the legislative process of conformity assessment. Strengthen the revision of departmental regulations and normative documents in the field of certification, accreditation, inspection and testing, and improve rules and regulations such as qualification of practitioners, management of employees, conformity assessment activities and supervision and management; Fully implement the requirements of legality review and fair competition review, and improve the quality of legislation.
(2) scientifically define the rights and responsibilities. Fully implement list management, establish a list of powers, a list of responsibilities and a negative list of market access in the field of certification, accreditation, inspection and supervision, and effectively regulate administrative law enforcement behavior and market subject behavior; Adhere to the principle of "who approves, who supervises, who is in charge and who supervises" and clarify the boundaries of industry management responsibilities; Adhere to the combination of territorial management and hierarchical management, and rationally divide the regulatory powers and responsibilities of certification, accreditation, inspection and testing at all levels.
(3) Improve the supervision and restriction mechanism. Strictly regulate the use of public power in the field of certification and accreditation inspection and testing, improve the supervision and restriction mechanism of power operation, earnestly strengthen the construction of a clean and honest government, strengthen the connection between discipline and execution, increase supervision, implement supervision responsibilities, and resolutely safeguard the fairness, integrity and effectiveness of certification and accreditation inspection and testing.
2. Strengthen technical support
(1) Optimize the supply of conformity assessment standards. Improve the standardization mechanism of conformity assessment, give full play to the centralized management function of the National Technical Committee for Certification and Accreditation Standardization (TC261), accelerate the transformation and application of international standards for conformity assessment, standardize and guide the coordinated development of national standards, industry standards and group standards in the field of conformity assessment, and form a more scientific and reasonable conformity assessment standard system. Actively promote the application of advanced standards and the latest scientific and technological achievements in the field of conformity assessment, carry out self-examination and clean-up of industry standards, actively adopt industry advanced standards, encourage industry associations and industrial technology alliances to develop group standards that meet the needs of conformity assessment, and comprehensively improve the supply level of conformity assessment standards.
(2) Improve the innovation ability of conformity assessment. Strengthen policy theory research, improve the top-level design of conformity assessment system, and provide policy guidance and intellectual support for the innovative development of certification, accreditation, inspection and testing; Improve the integration mechanism of Industry-University-Research, encourage practitioners to develop conformity assessment technical schemes higher than the general requirements of the industry, encourage enterprises, research institutes and industry associations to participate in conformity assessment innovation research and development activities, improve the "certification system owner" mechanism, and strengthen the protection and application of independent innovation technological achievements; We will tackle key core technologies, adapt to the development trend of conformity assessment technology from small sample sampling to big data analysis, from single attribute to multi-attribute system evaluation, from qualitative evaluation to quantitative evaluation, and from traditional technology to intelligent and full-life cycle technology, and carry out technical research on digitalization, intelligence, precision and systematization of conformity assessment, so as to comprehensively improve the supporting and leading ability of conformity assessment technology.
(3) Promote the high-level utilization of inspection and testing resources. Promote the high-end development of inspection and testing instruments and equipment, guide resources and funds to the research and development of inspection and testing instruments and equipment, strengthen the research and development and application of digital and intelligent testing technology and equipment, establish a verification and evaluation system for domestic inspection and testing instruments and equipment, carry out performance comparison, verification and comprehensive evaluation of high-end instruments and meters at home and abroad, and improve the technical level of inspection and testing instruments and equipment; Strengthen the development of standard samples/reference materials for inspection and testing, deepen the innovative research and development and intellectual property protection in inspection and testing methods and regulations, instruments and equipment, experimental environment and trademark identification, and improve the comprehensive application level of inspection and testing element resources; Construct an open sharing platform for inspection and testing resources to improve the efficiency of resource development and utilization.
3. Strengthen talent guidance
(1) Build an education and training base. Strengthen the education construction of certification and accreditation inspection and testing disciplines, support institutions of higher learning, vocational and technical schools, education and training institutions to strengthen cooperation with certification and accreditation inspection and testing institutions, build education and training demonstration bases, develop qualified assessment education and training courses to meet the needs of industry and society, and provide multi-level, multi-field and multi-channel professional training for industry, government and society.
(2) Improve the ability of professional teams. Establish and improve the professional qualification system of quality certification and inspection and testing practitioners, improve the ability evaluation and continuing education mechanism, and continuously improve the ability and quality of personnel. Strengthen the management of registration examination for certification practitioners, organize training activities in Certification staff, expand the team of certification auditors, strengthen the training and supervision of assessors in inspection and testing institutions, optimize the selection and on-site assessment mechanism of assessors, promote the construction of industry assessment teams, and improve the professional ethics and professional competence of assessors. Vigorously carry forward the entrepreneurial spirit and craftsman spirit, make the pursuit of Excellence and advocating quality become the value orientation of the industry, and create high-quality management and industry leaders of inspection and certification institutions.
(3) Strengthen the construction of supervision team. Strengthen the supervision capacity building of certification, accreditation, inspection and testing of market supervision departments at all levels, clarify the powers and responsibilities of supervision functions, enrich the allocation of supervision resources, equip the supervision staff, strengthen the inclined support for the grassroots front line, and build a certification, accreditation, inspection and testing supervision team with excellent politics, exquisite business and excellent work style. Strengthen training guidance, enrich and improve training materials and case base of supervision and law enforcement, carry out multi-level training exchanges, on-the-job training, skill competition and other activities to enhance the ability of supervision and law enforcement.
Fourth, safeguard measures
(1) Strengthen organizational leadership. Strengthen the party’s leadership over certification, accreditation, inspection and testing, and provide a strong political guarantee for the implementation of the plan. Establish a working system of unified leadership, departmental coordination and linkage from top to bottom, comprehensively strengthen overall coordination and organization and implementation, and create a good policy environment, market environment and rule of law environment. Give full play to the industry management function of CNCA, strengthen the role of CNCA in overall coordination, system scheduling, and guarantee implementation, and improve the planning implementation mechanism. We will improve the working mechanism of the national inter-ministerial joint conference on certification and accreditation, enhance the efficiency of inter-ministerial cooperation, and strengthen policy convergence, planning guidance and work coordination. Promote all departments and localities to incorporate certification, accreditation, inspection and testing into special plans and local plans, and guide enterprises, institutions, industry organizations and social organizations to actively participate in the implementation of the plan and form a joint effort. Local market supervision departments should combine the actual situation, formulate the planning and implementation plan, decompose and implement the objectives and tasks, clarify the responsible subjects, and ensure the implementation as scheduled.
(2) Improve investment guarantee. Encourage all departments and localities to formulate policies to promote certification, accreditation, inspection and testing and implement projects, establish and improve the capital investment guarantee mechanism combining government, enterprises and society, encourage and guide the standardized and orderly investment of financial funds and social capital, actively support local governments, enterprises and institutions to arrange matching funds for major projects, explore market-oriented capital channels such as development funds, project investment and financing, and quality liability insurance, give play to the synergistic effect of financial, taxation, financial and insurance tools, and increase investment support.
(3) Strengthen publicity and guidance. We will carry out in-depth theme activities such as "Quality Month", "World Accreditation Day" and "Open Day of National Inspection and Testing Institutions", give full play to the role of news media and new media platforms, strengthen the publicity, policy interpretation, knowledge popularization and achievement display of certification and accreditation inspection and testing, improve the quality awareness and integrity awareness of the whole society, and enhance market confidence. Actively publicize the excellent practice of conformity assessment in China, encourage practitioners to carry out international publicity and promotion activities, and enhance their international influence. Strengthen public opinion guidance, respond to public opinion trends in a timely manner, and create a good public opinion environment.
(4) Strictly implement the evaluation. According to the evaluation principle of combining target evaluation with process monitoring, we will monitor, evaluate and guide the implementation of the plan every year, study and solve new situations and problems in the implementation process in a timely manner, give full play to the constraint and leading role of the plan, and ensure the high-quality and efficient completion of the development objectives and tasks during the 14 th Five-Year Plan period.